
Ionic Liquids (ILs) are liquids at temperatures below 100ºC that are entirely made of ions. They typically consist of nitrogen-containing organic cations and inorganic anions. Typical cations are nitrogen substituted imidazolium and pyridinium cations. Typical anions are Cl–, Cl–/AlCl3, PF6– and BF4–. ILs are non-volatile and non-flammable and have high thermal stability. Most current organic solvents are volatile organic compounds (VOCs) that originate environmental, human health and safety concerns. ILs represent a positive alternative to the use of VOCs.
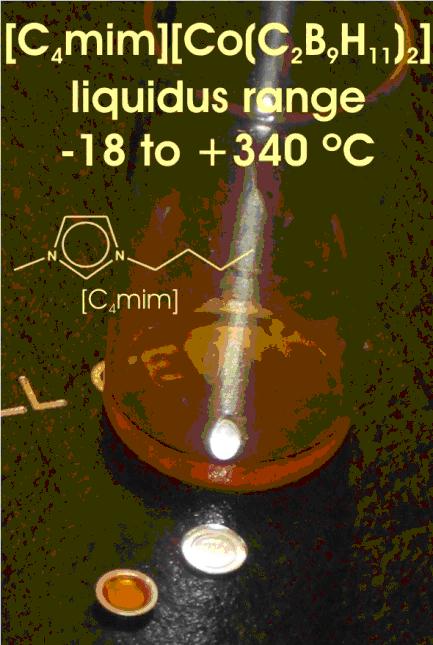
Their importance, however, is not only limited to their use as solvents to diminish the risks of VOCs. They offer new scientific and engineering possibilities. Perhaps the most relevant is the almost unlimited possibilities of generating new ILs with 1:1 compositions just by combining cations and anions to get an IL that suits the demanded properties. Ionic liquids are often composed of poorly coordinating ions, so they can find applications where such properties are needed either in syntheses or in technological applications most relevantly when electrolytes are necessary. ILs can be made immiscible with a number of organic solvents or water providing a non-aqueous, polar alternative for two-phase systems. When two phase systems are needed, ILs should be taken in consideration. As a consequence ILs are a real opportunity to enlarge enormously the possibilities of homogeneous catalysis in industrially attractive processes, and in separation processes. One of the goals of this group is the development of boron cluster chemistry along with its possible applications. Boron clusters having a closed structure, closo species, can be neutral, monoanionic and dianionic. Due to the charge delocalization on the cluster, mono and dianionic clusters have a low charge density, see that the negative charge in [Co(C2B9H11)2]– can be distributed among 45 atoms, thence they are good candidates to generate ILs. It is the interest of this group to generate ILs using the cations shown above or similar and closo anionic boron clusters.
ILs produce the highest possible concentration of ions, and this property in combination with the special features of anionic borane clusters may be of special relevance in electrosynthesis. Closo dianions are common in boron cluster chemistry. In principle dianions should be less weakly coordinating, more coordinating therefore, than monoanions, however due to the special structure and geometry of boron clusters we anticipate intriguing properties for these new [cat] 2 [Y] ILs where cat means the cations. To this aim research in this group is dedicated to get procedures for the high yield synthesis of [BnXn]2– species in order to explore the properties of this new type of ILs.
ILs produce the highest possible concentration of ions, and this property in combination with the special features of anionic borane clusters may be of special relevance in electrosynthesis. Closo dianions are common in boron cluster chemistry. In principle dianions should be less weakly coordinating, more coordinating therefore, than monoanions, however due to the special structure and geometry of boron clusters we anticipate intriguing properties for these new [cat] 2 [Y] ILs where cat means the cations. To this aim research in this group is dedicated to get procedures for the high yield synthesis of [BnXn]2– species in order to explore the properties of this new type of ILs.
References:
“Ionic liquids containing boron cluster anions”
Inorganic Chemistry, 2009, 48, 889
M. Nieuwenhuyzen, K. R. Seddon, F. Teixidor, A. Vaca, C. Viñas
