Author/s: A. V. Puga, F. Teixidor, R. Sillanpää, R. Kivekäs and C. Viñas
Reference: Chemistry – A European Journal, 2009, 15, 9764-9772.
Abstract:
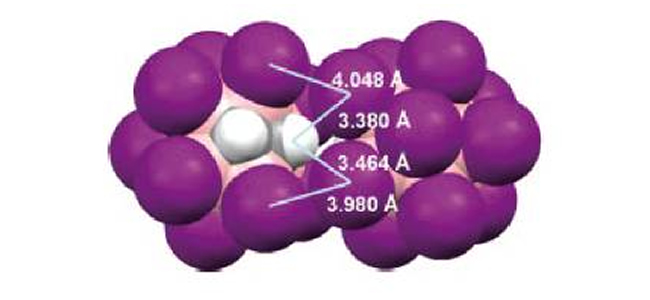
The crystal structures of numerous iodinated ortho-carboranes have been studied, which has revealed the diversity of intermolecular interactions that these substances can adopt in the solid state. The nature-mostly as it relates to hydrogen and/or halogen bonds- and relative strength of such interactions can be adjusted by selectively introducing substituents onto the cluster, thus enabling the rational design of crystal lattices. In this work we present the newly determined crystal structures of the following iodinated ortho-carboranes: 9-I-1,2-closo-C2B10H11, 4,5,7,8,9,10,11,12-I8-1,2-closo-C2B10H4, 3,4,5,6,7,8,9,10,11,12-I10-1,2-closo-C2B10H2, 1-Me-8,9,10,12-I4-1,2-closo-C2B10H7, 1,2-Me2-8,9,10,12-I4-1,2-closo-C2B10H6, and 1,2-Ph2-8,9,10,12-I4-1,2-closo-C2B10H6. Their 3D supramolecular organization has been thoroughly investigated and compared to similar previously published crystal structures.
Such a systematic survey has allowed us to draw some general trends. Cc-H···I-B hydrogen bonds (Cc = cluster carbon atoms) appear to be significant in the growth of the crystal lattices of these compounds, given the acidity of hydrogen atoms bonded to Cc, and the polarization of B-I bonds. These hydrogen bonds can be disrupted by selectively blocking the positions next to Cc, that is, B(3) and B(6), with bulky substituents that prevent iodine atoms from approaching as hydrogen acceptors. Halogen bonds of the type B-I···I-B are frequently observed in most cases, thus suggesting that these interactions could be attractive in boron clusters. In addition, different substituents can be grafted onto the ortho-carborane surface, thereby providing further possibilities for homomeric or heteromeric molecular assembly.
