Ion recognition, thus chemical sensors based on Ion Selective Electrodes, or ISFET’s, Ion Selective Field Effect Transistors; transport membranes based on Supported Liquid Membranes (SLM’s) and Fixed Site Complexing Membranes (FSCM’s).
Cyclic voltammetry of PPy[Co(C2B9H11)2] in aqueous alkaline chlorides 0.1 M: LiCl, NaCl, KCl, RbCl, CsCl.
The high volume of [Co(C2B9H11)2]– imposes low mobility inside the polymeric matrix thus preventing dopant leakage when a reducing potential is applied on the material. Under these conditions the cation capture (Eq. 1) prevails during the reversible electrochemical redox process, also called doping/undoping. The membrane is highly sensitive to the cationic volume of the solute. This property has allowed us to develop cationic selective membranes of Li+, Na+, K+ and Rb+ by control of the applied reducing potential (Fig. 1). The chronocoulometries registered during the charge-discharge process show an almost perfect reversibility of cation exchange process and no detectable degradation of the membrane due to dopant loss or overoxidation even after 40 successive cycles.
[PPyn + (A–)n] + nC+ + ne– → [PPy(A–)n(C+)n] (Equation1)The electrode is suitable for pH measurements: Monoprotic titrations of strong alkalis with strong acids, and weak bases with strong acids.
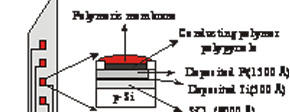
This property has been applied to manufacture of hydrogen-selective microelectrodes on silicon needle-shaped substrates . The performance of the resulting solid-contact ion-selective microelectrodes (SCISME) was investigated by using potentiometric measurement and electrochemical impedance spectrometry. The feasibility of the fabrication technology is demonstrated and the devices operate satisfactorily, with a response showing good sensitivity and selectivity against common interfering cations in background solutions. The SCISME has been developed for organ monitoring during cardiac surgery or during transportation for transplants.
Projects:
“Síntesis de Metalocarboranos Substituídos. Estudio de su actividad como transportadores en membranas líquidas”. (PB94/0226).
“Materiales poliméricos Polipirrol/Metalacarboranos para purificación de aguas y separación de gases” (MAT98-0921).
References:
[1] C. Masalles, S. Borros, C. Viñas, F. Teixidor, Advanced Materials, 2000, 12, 1199 – 1202.[2] C. Masalles, F. Teixidor, S. Borrós, C. Viñas, Journal of Organometallic Chemistry, 2002 , 657, 239 – 246.
[3] C. Masalles, S. Borrós, C. Viñas, F. Teixidor, Analitical and Bioanalytical Chemistry, 2002, 372, 513 – 518.
[4] N. Zine, J. Bausells, A. Ivorra, J. Aguiló, M. Zabala, F. Teixidor, C. Masalles, C. Viñas, A. Errachid, Sensors and Actuators B, 2003, 91, 76 – 82.
